Track your food intake exercise sleep and meditation for free. Molar mass of C6H12O6 glucose is 1801559 gmol Get control of 2022.

What Is The Molar Mass Of Glucose C6h12o6 Lisbdnet Com
Molar mass will be the mass of the substance in grams.

. Calculate the molar mass of a gas produced when 0120 g of its liquid are vaporized at a temperature of 50 C and occupy a volume of. Since it is mass of empirical formula. The molar mass of C6H12O6 can be calculated by the sum total of the molar masses of its elements as follow.
Not at all Slightly Kinda Very much Completely. See the answer See the answer done loading. Now click the button Calculate Molar Mass to get the result.
Multiply the atomic weight of each element with the number of atoms of that particular element. Advertisement Survey Did this page answer your question. Molar mass of C 1201 gmol x 6 7206 gmol Molar mass of H 1008 gmol x 12 12096 gmol.
2 Get Another question on Chemistry. Molar mass of C6H12O6 18016 g b. Note the molar mass found in this video is 18018 gmol.
A few things to consider when find. Identify the formula of the compound or molecule. Hydrogen H 1 gmol Molar mass of C₆H₁₂O₆ glucose 6 12 12 1 6 16 108gmol.
The molar mass of C6H12O6 is 18018 gmol What is the molar mass of glucose. 2 question Calculate the molar concentration of a solution containing 433 grams of C6H12O6 in 82 milliliters of water. A S8 b C5H12 c SC2 SO43 d CH3COCH3 acetone e C6H12O6 glucose 2.
Experts are tested by Chegg as specialists in their subject area. What is the molar mass of C6H12O6. 3 rows Molar Mass Molecular Weight and Elemental Composition Calculator Enter a chemical formula to.
Calculate the molar mass of glucose C 6 H 12 O 6. 6x12 12x1 6x16. So the molar mass of C6H12O6 is 7206 g 12096 g 9600 g 180156 g or 18016 g right number of significant figures.
Enter the chemical compound in the respective input field. The unit cell of a compound containing Co and O. And the empirical formula of glucose is CH2O.
Two moles one mole. Get 1-on-1 help from an expert tutor now. 4 rows Molar mass of C6H12O6 18015588 gmol.
180g will be the molecular mass of the glucose molecule. 4 rows The molar mass of C6H12O6 glucose is. Finally the molar mass of.
This compound is also known as Glucose or Fructose or. 1x12 2x1 1x16. Explanation of how to find the molar mass of C6H12O6 Glucose.
Moles of CaCO3 taken mass molar mass of CaCO3 321 100 0321 moles of HCl taken molarit. A 250 g of propylene C3H6 b 306 x 10-3 g of the amino acid glycine C2H5NO2 c 25 lb of the herbicide. Molecular mass of glucose C6H12O6 6 12 1 12 6 16 72 12 96 180 g of carbon C in glucose 72 180 100 40 of hydrogen H in glucose 12 180 100 666 of oxygen O in glucose 96 180 100 5333.
The molar mass of C6H12O6 is 180156 gmol. Determine the number of moles of compound and the number of moles of each type of atom in each of the following. What is the mass of 131 1022 molecules of glucose.
Using the formula to determine the number of atoms present in each element of the compound or molecule. How do you find the molar mass of C6H12O6 ie. Consider a 1245g sample of glucose C6H12O6.
Formula Unit Mass-. 1Calculate the molar mass of each of the following. Prev Question Next Question.
Convert between C6H12O6 glucose weight and moles Elemental composition of C6H12O6 glucose Formula in Hill system is C6H12O6 Computing molar mass molar weight. The number of moles 469g 180156gmol 26mol Volume of water in litres 821000 0082L Concentration of solution 26mol 0082L 3171M. Molecular mass of C6H12O6 12 Atomic mass of Hydrogen 6 Atomic mass of carbon 6 Atomic mass of oxygen 12 x 1 6X12 6X16 12 72 96 180 u.
One mole of water contains _____ of hydrogen atoms and _____ of oxygen atoms. Calculation of Molar mass of a compound. Using the periodic table the atomic weight of C H and O are obtained.
We review their content and use your feedback to keep the quality high. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. It formula unit mass will be.
Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. Who are the experts. See also our theoretical yield.
A S8 b C5H12 c Sc2SO43 d CH3COCH3 acetone e C6H12O6 glucose Answer a 256528 gmol Answer b 72150 gmol Answer c 378103 gmol Answer d 58080 gmol Answer e 180158 gmol PROBLEM PageIndex9 Calculate the molar mass of each of the following. According to this question there are 469 grams of C6H12O6 in 82 milliliters of water. PROBLEM PageIndex8 Calculate the molar mass of each of the following.
Molar mass of of the atoms are - Carbon C 12 gmol oxygen O 16 gmol. Chemistry 22062019 0010.

Molar Mass Molecular Weight Of C6h12o6 Glucose Youtube

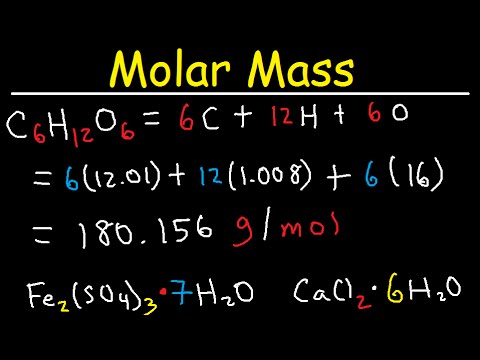
0 Comments